NHAI Explores Use of Waste Materials for Ecologically Sustainable National Highway Infrastructure
NHAI and the Department of Fertilizers are conducting field trials to explore the use of Phosphor-Gypsum in National Highway construction to achieve a circular economy in the use of gypsum.
This by-product of fertilizer production was accredited by the Indian Road Congress for road construction for three years based on evaluations conducted by CRRI.
NHAI also encourages the use of waste plastic and fly ash in road construction as they have been proven to be durable, sustainable and economical. These initiatives are part of NHAI's commitment to reducing carbon footprint and promoting ecologically sustainable National Highway Infrastructure.
Phosphor-Gypsum
What is Phosphor-Gypsum?
Phosphor-gypsum is a by-product of the phosphate fertilizer industry, which is produced during the wet process of phosphoric acid production. It is a type of gypsum that contains high levels of phosphorus and other impurities, making it unsuitable for use in construction.
Composition and Characteristics:
Phosphor-gypsum is a white, powdery substance that is similar in appearance to regular gypsum. It typically contains between 10% and 25% phosphorus pentoxide (P2O5), as well as other impurities such as fluoride, silica, and heavy metals. Phosphor-gypsum is also slightly more soluble in water than regular gypsum, which can lead to environmental concerns if it is not properly disposed of.
Uses and Applications:
Phosphor-gypsum is not suitable for use in construction due to its high levels of impurities. However, it can be used as a soil amendment to improve the fertility of agricultural land. The phosphorus in phosphor-gypsum is readily available to plants, making it a valuable fertilizer. Additionally, phosphor-gypsum can be used in cement production as a substitute for natural gypsum. This can help reduce the environmental impact of cement production, as natural gypsum is a finite resource.
Environmental Concerns:
Phosphor-gypsum can pose environmental concerns if it is not properly disposed of. The high levels of phosphorus in phosphor-gypsum can lead to eutrophication of water bodies if it is discharged into rivers or lakes. Eutrophication occurs when excess nutrients, such as phosphorus, enter a body of water and stimulate the growth of algae and other aquatic plants. This can lead to oxygen depletion and the death of fish and other aquatic life.
Regulations:
In many countries, regulations have been put in place to control the disposal of phosphor-gypsum. For example, in the European Union, phosphor-gypsum is classified as a waste and must be disposed of in accordance with the Waste Framework Directive. In the United States, the Environmental Protection Agency (EPA) has established guidelines for the management of phosphor-gypsum, which include requirements for storage, transportation, and disposal.
Recently, scientists have discovered a third natural source of quasicrystals at the Sand Hills of north central Nebraska, USA.
Quasicrystals and their unique properties:
Have you ever wondered why some crystals have perfectly symmetrical shapes and patterns, while others look like a jumbled mess? The answer lies in their atomic structure. Most crystals have a repeating pattern of atoms, known as a lattice, that gives them their regular shape. But there's a rare type of crystal that breaks this rule, called a quasicrystal.
Quasicrystals were first discovered in 1982 by the Israeli mathematician and crystallographer, Dan Shechtman. At first, he was met with skepticism by the scientific community, who believed that such a crystal structure was impossible. But Shechtman persisted and eventually won the Nobel Prize in Chemistry in 2011 for his discovery.
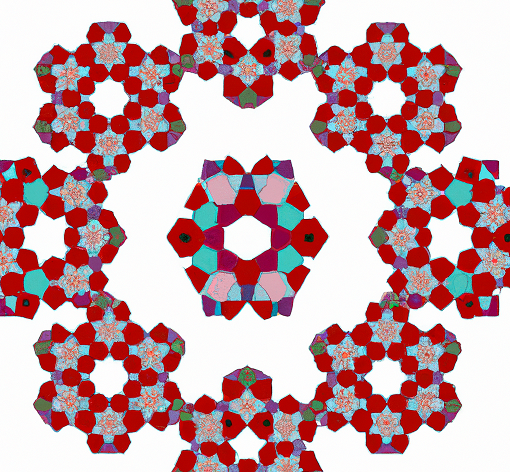
What makes quasicrystals so unique is that they have a non-repeating pattern that never quite repeats itself, known as a Penrose pattern. This gives them a strange, yet beautiful appearance, with sharp edges and intricate shapes that seem to defy the laws of geometry.
Unlike regular crystals, which have a fixed number of symmetries, quasicrystals have infinite symmetries. This means that no matter how many times you rotate or reflect a quasicrystal, it will always look the same. This property is known as "quasiperiodicity," and it's what makes quasicrystals so fascinating to scientists and mathematicians.
Quasicrystals are not just aesthetically pleasing, but they also have unique physical properties. They are extremely hard and can resist corrosion and wear, making them ideal for use in coatings and industrial applications. They also have low friction, which makes them useful in bearings and other moving parts.
Researchers are still trying to uncover the full potential of quasicrystals and their applications in various fields, from electronics to medicine. They are also studying the natural occurrence of quasicrystals in minerals, such as the mineral called icosahedrite, which was discovered in Russia in 1992.
Quasicrystals are a fascinating and mysterious world of non-repeating patterns that defy the traditional rules of crystallography. With their unique physical properties and infinite symmetries, they have the potential to revolutionize many areas of science and technology. And who knows, maybe one day we'll even discover new quasicrystals that are even more bizarre and beautiful than we could ever imagine.
6-Feb-2023: Scientists at UCL created a new type of ice
Scientists at University College London (UCL) have recently created a new type of ice that matches the density and structure of water. The ice is called medium-density amorphous ice.
Properties:
- Matches density and structure of water
- Created by shaking regular ice in a container with steel balls at a temperature of -200°C
- Appeared as white granular powder that stuck to the metal balls.
Ice is less dense than liquid form due to their unusual property. Water can solidify in many regular arrangements depending on conditions such as pressure and the speed of freezing. Amorphous ice, however, is different as it has no such order. Hence, this study could probably help in studying water’s mysterious properties.